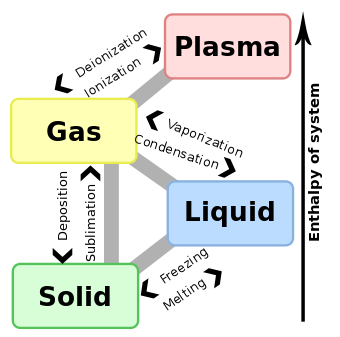
Enthalpy of vaporization
.png)
The enthalpy of vaporization, (symbol 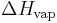 ), also known as the heat of vaporization or heat of evaporation, is the energy required to transform a given quantity of a substance into a gas at a given pressure (often atmospheric pressure).
), also known as the heat of vaporization or heat of evaporation, is the energy required to transform a given quantity of a substance into a gas at a given pressure (often atmospheric pressure).
It is often measured at the normal boiling point of a substance; although tabulated values are usually corrected to 298 K, the correction is often smaller than the uncertainty in the measured value.
The heat of vaporization is temperature-dependent, though a constant heat of vaporization can be assumed for small temperature ranges and below Tr<<1.0. The heat of vaporization diminishes with increasing temperature and it vanishes completely at the critical temperature (Tr=1) because above the critical temperature the liquid and vapor phases no longer co-exist.
Contents |
Units
Values are usually quoted in J/mol or kJ/mol (molar enthalpy of vaporization), although kJ/kg or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used, among others.
Physical model for vaporization
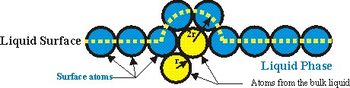
A simple physical model for the liquid-gas phase transformation has been proposed recently [1] . It is suggested that the energy required to free an atom from the liquid is equivalent with the energy needed to overcome the surface resistance of the liquid. The model allows calculating the latent heat by multiplying the maximum surface area covering an atom (Fig. 1) with the surface tension and the number of atoms in the liquid. The calculated latent heat of vaporization values for the investigated 45 elements agrees well with experiments.
Enthalpy of condensation
The enthalpy of condensation (or heat of condensation) is numerically exactly equal to the enthalpy of vaporization, but has the opposite sign: enthalpy changes of vaporization are always positive (heat is absorbed by the substance), whereas enthalpy changes of condensation are always negative (heat is released by the substance).
Thermodynamic background
The enthalpy of vaporization can be viewed as the energy required to overcome the intermolecular interactions in the liquid (or solid, in the case of sublimation). Hence helium has a particularly low enthalpy of vaporization, 0.0845 kJ/mol, as the van der Waals forces between helium atoms are particularly weak. On the other hand, the molecules in liquid water are held together by relatively strong hydrogen bonds, and its enthalpy of vaporization, 40.65 kJ/mol, is more than five times the energy required to heat the same quantity of water from 0 °C to 100 °C (cp = 75.3 J K−1 mol−1). Care must be taken, however, when using enthalpies of vaporization to measure the strength of intermolecular forces, as these forces may persist to an extent in the gas phase (as is the case with hydrogen fluoride), and so the calculated value of the bond strength will be too low. This is particularly true of metals, which often form covalently bonded molecules in the gas phase: in these cases, the enthalpy of atomization must be used to obtain a true value of the bond energy.
An alternative description is to view the enthalpy of condensation as the heat which must be released to the surroundings to compensate for the drop in entropy when a gas condenses to a liquid. As the liquid and gas are in equilibrium at the boiling point (Tb), ΔvG = 0, which leads to:
As neither entropy nor enthalpy vary greatly with temperature, it is normal to use the tabulated standard values without any correction for the difference in temperature from 298 K. A correction must be made if the pressure is different from 100 kPa, as the entropy of a gas is proportional to its pressure (or, more precisely, to its fugacity): the entropies of liquids vary little with pressure, as the compressibility of a liquid is small.
These two definitions are equivalent: the boiling point is the temperature at which the increased entropy of the gas phase overcomes the intermolecular forces. As a given quantity of matter always has a higher entropy in the gas phase than in a condensed phase (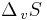 is always positive), and from
is always positive), and from
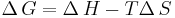 ,
,
the Gibbs free energy change falls with increasing temperature: gases are favored at higher temperatures, as is observed in practice.
Selected values
Elements
Enthalpies of vaporization of the elements in kJ/mol, measured at their respective normal boiling points
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ Period | ||||||||||||||||||||
| 1 | H 0.44936 |
He 0.0845 |
||||||||||||||||||
| 2 | Li 145.92 |
Be 292.40 |
B 489.7 |
C 355.8 |
N 2.7928 |
O 3.4099 |
F 3.2698 |
Ne 1.7326 |
||||||||||||
| 3 | Na 96.96 |
Mg 127.4 |
Al 293.4 |
Si 300 |
P 12.129 |
S 1.7175 |
Cl 10.2 |
Ar 6.447 |
||||||||||||
| 4 | K 79.87 |
Ca 153.6 |
Sc 314.2 |
Ti 421 |
V 452 |
Cr 344.3 |
Mn 226 |
Fe 349.6 |
Co 376.5 |
Ni 370.4 |
Cu 300.3 |
Zn 115.3 |
Ga 258.7 |
Ge 330.9 |
As 34.76 |
Se 26.3 |
Br 15.438 |
Kr 9.029 |
||
| 5 | Rb 72.216 |
Sr 144 |
Y 363 |
Zr 581.6 |
Nb 696.6 |
Mo 598 |
Tc 660 |
Ru 595 |
Rh 493 |
Pd 357 |
Ag 250.58 |
Cd 100 |
In 231.5 |
Sn 295.8 |
Sb 77.14 |
Te 52.55 |
I 20.752 |
Xe 12.636 |
||
| 6 | Cs 67.74 |
Ba 142 |
* |
Hf 575 |
Ta 743 |
W 824 |
Re 715 |
Os 627.6 |
Ir 604 |
Pt 510 |
Au 334.4 |
Hg 59.229 |
Tl 164.1 |
Pb 177.7 |
Bi 104.8 |
Po 60.1 |
At 114 |
Rn 16.4 |
||
| 7 | Fr n/a |
Ra 37 |
** |
Rf n/a |
Db n/a |
Sg n/a |
Bh n/a |
Hs n/a |
Mt n/a |
Ds n/a |
Rg n/a |
Cn n/a |
Uut n/a |
Uuq n/a |
Uup n/a |
Uuh n/a |
Uus n/a |
Uuo n/a |
||
| * Lanthanides | La 414 |
Ce 414 |
Pr n/a |
Nd n/a |
Pm n/a |
Sm n/a |
Eu n/a |
Gd n/a |
Tb n/a |
Dy n/a |
Ho n/a |
Er n/a |
Tm n/a |
Yb n/a |
Lu n/a |
|||||
| ** Actinides | Ac n/a |
Th 514.4 |
Pa n/a |
U n/a |
Np n/a |
Pu n/a |
Am n/a |
Cm n/a |
Bk n/a |
Cf n/a |
Es n/a |
Fm n/a |
Md n/a |
No n/a |
Lr n/a |
|||||
| 0–10 kJ/mol | 10–100 kJ/mol | 100–300 kJ/mol | >300 kJ/mol |
Other common substances
Enthalpies of vaporization of common substances, measured at their respective normal boiling points:
| Compound | Heat of vaporization (kJ mol-1) |
Heat of vaporization (kJ kg-1) |
|---|---|---|
| Ammonia | 23.35 | 1371 |
| Butane | 21.0 | 320 |
| Ethanol | 38.6 | 841 |
| Hydrogen | 0.46 | 451.9 |
| Methane | 8.19 | 760 |
| Methanol | 35.3 | 1104 |
| Propane | 15.7 | 356 |
| Phosphine | 14.6 | 429.4 |
| Water | 40.65 | 2257 |
Water is also commonly expressed as 539.423 calories per gram
See also
- Enthalpy of fusion
- Enthalpy of sublimation
- Joback method (Estimation of the heat of vaporization at the normal boiling point from molecular structures)
References
- ↑ doi:10.1016/j.fluid.2009.06.005
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand
Sears, Zemansky et al., University Physics, Addison-Wesley Publishing Company, Sixth ed., 1982, ISBN 0-201-07199-1
NIST Chemistry WebBook CODATA Key Values for Thermodynamics
|
|||||||||||||||||||||||||